LEMAR’s NOVEL BIODIESEL PRODUCTION PROCESS
LeMar has developed novel processes for biodiesel production for all stages. It is provides highest economic efficiency for edible and non-edible oils and especially for waste cooking oil.
Benefits:
- Allows flow process with quick reaction and glycerol separation in liquid form.
- Saves about 90% of energy needed in comparison with the old batch type technology.
- Saves on materials use with more precise mixing and recycling.
- Uses a lot less water and recycles it.
- Utilizes waste into biogas and fertilizer.
A. TREATMENT OF WASTE COOKING OIL.

Fig. 1.
- This process allows quick and easy separation in centrifuge. Refined oil is ready for transesterification.
- This process allows to prepare waste cooking with up to 5% of FFA for transesterification.
- The removed solids are good for bio processing into bio methane and can be processed together with crude glycerol from the transesterification process.
B. BIODIESEL PRODUCTION PROCESS.
LeMar developed novel in-flow process, which can be done in one stage for separated oil with FFA below 5% or in two stages above 5%.
Novel Transesterification.
The main biodiesel technology is transesterification is traditionally batch type process with 8 to 10 hours process time and a lot of energy used for mixing in large vessels. LeMar offer novel ultrasonic process and equipment to make it flow type process. It will lower energy consumption by 70% and the production cost by 40%.
The ultrasonic processing is a very desirable tool for producing biodiesel from vegetable oil and animal fats, because it lowers the cost of processing, speeds up transesterification, does not require elevated temperatures, and produces a higher grade of biodiesel.
Advantages are:
- With ultrasonic process, the amount of catalyst required for the transesterification of oil to biodiesel is substantially reduced.
- Ultrasonic processing is fast, usually minutes, compared to one hour or more using conventional batch reactor systems.
- Time required for glycerin phase separation is greatly reduced – typically less than 30 minutes instead of 4 to 8 hours in batch processing.
- Biodiesel yield is typically more than 95%.
- Ultrasonic processors generate non-inertial cavitation.
- Energy saving is 80% vs. batch processing.
Low (up to 5%) FFA Oil to Biodiesel Process Diagram
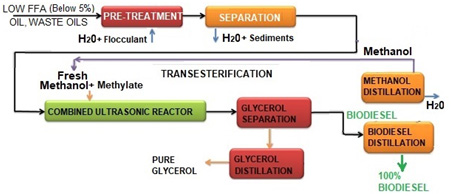
Fig.3. Low FFA process
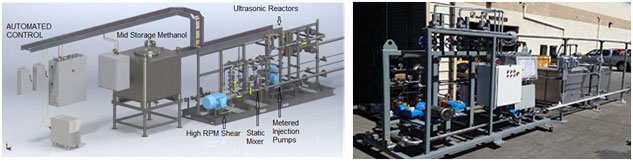
Fig.4. Biodiesel Production Line

